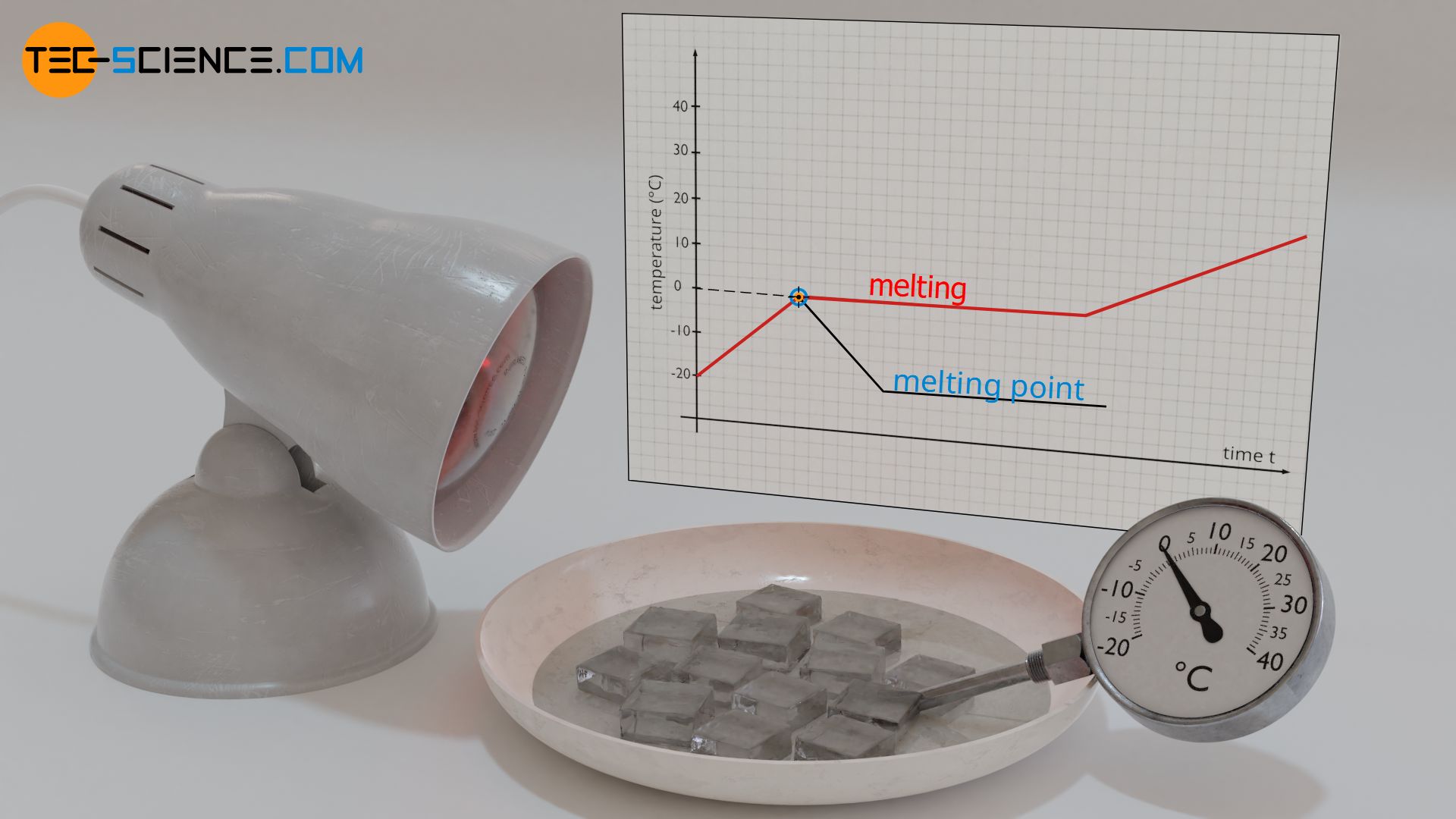
Why Does Temperature Increase With Pressure . The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This is a temperature scale where temperature and pressure are. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pressure is created by the number of collisions that occur between the molecules and the surface of container. Charles's law states that the volume of a. This law states that the pressure (p) of a fixed mass of gas held at a. If the temperature in the container.
from garciaspeausell.blogspot.com
We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This law states that the pressure (p) of a fixed mass of gas held at a. Charles's law states that the volume of a. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pressure is created by the number of collisions that occur between the molecules and the surface of container. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and pressure are. If the temperature in the container.
Why Did the Temperature Rise to a Constant Temperature Instead of
Why Does Temperature Increase With Pressure This law states that the pressure (p) of a fixed mass of gas held at a. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Charles's law states that the volume of a. Pressure is created by the number of collisions that occur between the molecules and the surface of container. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This law states that the pressure (p) of a fixed mass of gas held at a. If the temperature in the container. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and pressure are.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free Why Does Temperature Increase With Pressure We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Charles's law states that the volume of a. The energy added as work during the compression. Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID4200029 Why Does Temperature Increase With Pressure If the temperature in the container. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). Pressure is created by the number of collisions that occur between the molecules and the surface of container. This is a temperature scale. Why Does Temperature Increase With Pressure.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Why Does Temperature Increase With Pressure This is a temperature scale where temperature and pressure are. If the temperature in the container. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This law states that the pressure (p) of a fixed mass of gas held at a. The relationships among the volume of. Why Does Temperature Increase With Pressure.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Why Does Temperature Increase With Pressure This is a temperature scale where temperature and pressure are. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. If the temperature in the container. The energy added as work during the compression of a gas leads to an increase in pressure and temperature.. Why Does Temperature Increase With Pressure.
From garciaspeausell.blogspot.com
Why Did the Temperature Rise to a Constant Temperature Instead of Why Does Temperature Increase With Pressure We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This is a temperature scale where temperature and pressure are. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This law states that the pressure (p) of a fixed mass of gas. Why Does Temperature Increase With Pressure.
From www.researchgate.net
Temperature variation with height according to the U.S. Standard Why Does Temperature Increase With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Charles's law states that the volume of a. If the temperature in the container. If you had a way to increase pressure. Why Does Temperature Increase With Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Why Does Temperature Increase With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If the temperature in the container. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This law states that the pressure (p). Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Why Does Temperature Increase With Pressure If the temperature in the container. This law states that the pressure (p) of a fixed mass of gas held at a. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. This is a temperature scale where temperature and pressure are. The relationships among the volume of. Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Characteristics of the Atmosphere PowerPoint Presentation, free Why Does Temperature Increase With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Charles's law states that the volume of a. This is a temperature scale where temperature and pressure are. If the temperature in the container. We find that temperature and pressure are linearly related, and if the temperature is. Why Does Temperature Increase With Pressure.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Why Does Temperature Increase With Pressure This is a temperature scale where temperature and pressure are. This law states that the pressure (p) of a fixed mass of gas held at a. Pressure is created by the number of collisions that occur between the molecules and the surface of container. If you had a way to increase pressure with no volume change, then yes, temperature would. Why Does Temperature Increase With Pressure.
From mungfali.com
Maxwell Boltzmann Distribution Temperature Why Does Temperature Increase With Pressure This law states that the pressure (p) of a fixed mass of gas held at a. Charles's law states that the volume of a. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This is a temperature scale where temperature and pressure are. If. Why Does Temperature Increase With Pressure.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Why Does Temperature Increase With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t. Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID4874125 Why Does Temperature Increase With Pressure This law states that the pressure (p) of a fixed mass of gas held at a. Pressure is created by the number of collisions that occur between the molecules and the surface of container. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If the temperature in the container. A. Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Why Does Temperature Increase With Pressure If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. This law states that the pressure (p) of a fixed mass of gas held at a. Charles's law states. Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint Why Does Temperature Increase With Pressure This law states that the pressure (p) of a fixed mass of gas held at a. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If the temperature in the container.. Why Does Temperature Increase With Pressure.
From courses.lumenlearning.com
9.2 The Temperature of Earth’s Interior Physical Geology Why Does Temperature Increase With Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Charles's law states that the volume of a. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This is a temperature scale where temperature and pressure are. Pressure is created by the number of collisions that occur between. Why Does Temperature Increase With Pressure.
From www.nagwa.com
Question Video Determining How a Change in Temperature Affects the Why Does Temperature Increase With Pressure If the temperature in the container. This is a temperature scale where temperature and pressure are. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). We find that temperature and pressure are linearly related, and if the temperature. Why Does Temperature Increase With Pressure.
From www.slideserve.com
PPT Section 11.2 Properties of the Atmosphere PowerPoint Why Does Temperature Increase With Pressure This is a temperature scale where temperature and pressure are. Charles's law states that the volume of a. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Pressure is created by the number of collisions. Why Does Temperature Increase With Pressure.